Echolight®
What is Echolight?
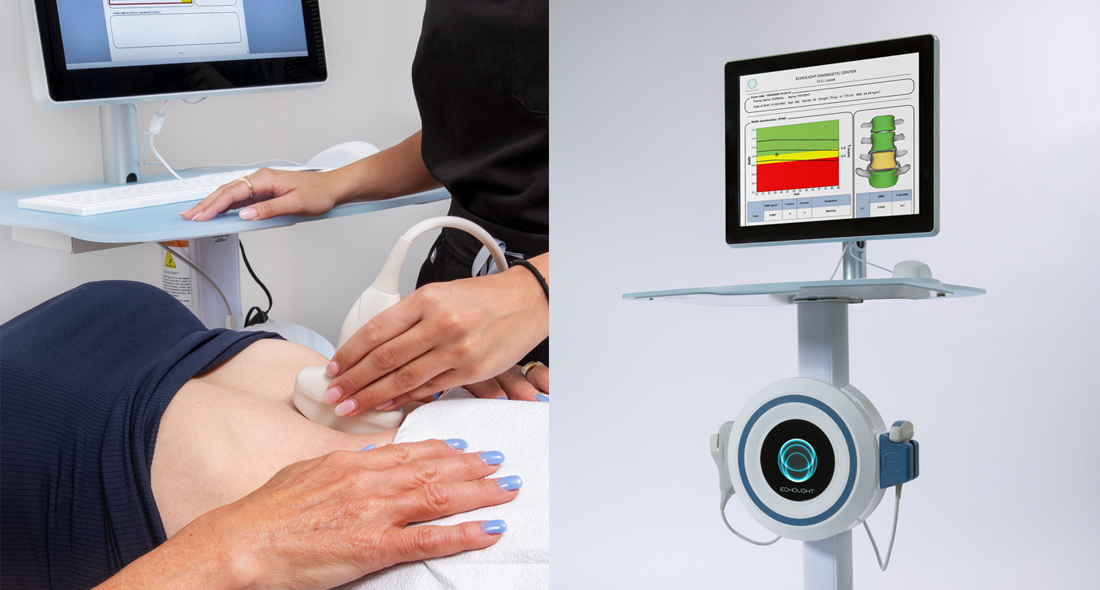
Echolight® is an FDA-approved, radiation-free bone density scan that is an alternative to the Dual-Energy X-Ray Absorptiometry (DEXA) scan. Developed in Italy and used throughout Europe for over a decade, Echolight uses Radiofrequency Echographic Multi Spectrometry (REMS Technology) to provide a more comprehensive and accurate assessment to evaluate bone health and bone density compared to the DEXA Scan.
While the DEXA scan remains the most commonly used tool for measuring bone mineral density (BMD), it does have notable limitations.
“A study from 2019 found that 90% of DEXA scans had technical errors. Interpretation errors were present in 80% of patients; 42% major. The most common major errors were reporting incorrect information on bone mineral density change (70%) and incorrect diagnosis (22%).”
Echolight combines radiofrequency technology with echographic (ultrasound) capabilities, creating a unique synergy that enables it to provide a multi-dimensional analysis of your bone properties. This integration of radiofrequency and echographic modalities allows Echolight to capture not only bone density measurements but also a wide range of additional information related to bone structure, composition, and health.
Unique to Echolight, the device provides a comprehensive assessment of bone architecture to predict fracture risk. The total assessment score is referred to as the “Fragility Score.” This is accomplished by combining consistent bone density scores with bone architecture analysis data.
Because Echolight does not use radiation, it is a great option to assess bone status in children, women of childbearing age, and/or those who are pregnant. Its use is also advantageous for individuals who require frequent monitoring of their bone health without the risk of cumulative radiation exposure such as cancer patients who may require regular monitoring of their bone density. Its precision is also valuable in diagnosing secondary osteoporosis conditions